Overview
Questions around matrix and elemental compatibility are encountered by all of us on a regular basis. This section addresses this question. Only the most popular matrices will be presented here. Consult our Interactive Periodic Table for other matrices and more information on compatibilities. Technique and calculations will be discussed in part 3 of this guide.
Nitric Acid Matrices
Most analysts prefer nitric acid (HNO3) matrices due to the solubility of the nitrates as well as its oxidizing ability and the relative freedom from chemical and spectral interferences as compared to acids containing Cl, S, F, or P. In addition, HNO3 is very popular in acid digestion sample preparations.
The elements that are stable/soluble and commonly diluted in aqueous/HNO3 are shaded in red below:

- Os should never be mixed with HNO3 due to the formation of the very volatile OsO4.
- Cl is oxidized to molecular Cl2 which is volatile and adsorbs on plastic.
- Br and I are oxidized to molecular Br2 and I2 which adsorb onto plastic.
- Dilutions of Hg and Au in HNO3 below 100 ppm should be stored in borosilicate glass due to Hg+2 adsorption on plastic.
- Not soluble above concentrations of 1000 µg/mL.
- Trace levels of HCl or Cl- will form AgCl, which will photoreduce to Ag0.
Hydrochloric Acid Matrices
The use of hydrochloric acid (HCl) is the next most popular acid matrix. HCl is volatile and it is corrosive to the instrument and it's electronics therefore, exposure should be kept to a minimum.
The elements that can be diluted in HCl are shaded in blue below:
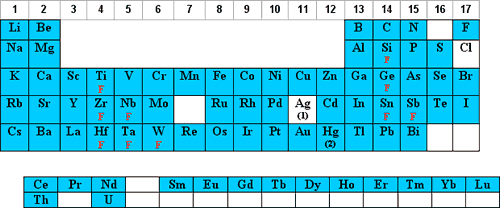
- Concentrated (35%) HCl will keep up to 100 µg/mL of Ag+ in solution as the Ag(Cl)X-(X-1) complex. For more dilute solutions, the HCl can be lowered such that 10% HCl will keep up to 10 µg/mL Ag in solution.
NOTE: The Ag(Cl)X-(X-1) complex is photosensitive and will reduce to Ag0 when exposed to light. HNO3 solutions of Ag+ are not photosensitive. - Parts-per-billion (ppb) dilutions of Hg+2 in HCl are more stable to adsorption on the container walls than are dilutions in HNO3.
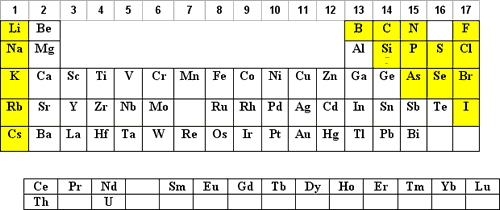
Water at pH of 7
Dilutions in water at pH 7 are not as common for most elements but may be required to prevent chemical reactions of some of the compounds containing the element. Please note that solutions at pH 7 may support biological growth and therefore the long-term stability should be questioned.
Those elements that may have an advantage to being diluted in water at pH 7 are shaded in yellow below:
Hydrofluoric Acid Matrices
Hydrofluoric acid (HF) requires the use of HF-resistant introduction systems. These systems are more expensive than glass, have longer washout times, and give a larger measurement precision. However, there are times when the use of HF offers a major advantage over other reagents.
Those elements where an HF matrix may be optimal are shaded in green below:
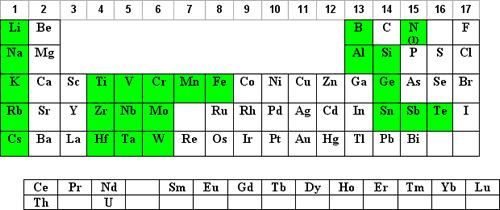
- HF is used for Si3N4 preparations and other nitrides.
Sulfuric Acid Matrices
Sulfuric acid (H2SO4) is commonly used in preparations and therefore added to standards in combination with other acids.
Elements that either benefit or comfortably tolerate the presence of H2SO4 are shaded in orange below:

- Dilutions of Hg and Au in H2SO4 below 100 ppm should be stored in borosilicate glass due to adsorption on plastic.
- Trace levels of HCl or Cl- will form AgCl, which will photoreduce to Ag0.
Phosphoric Acid Matrices
Phosphoric acid (H3PO4) is not commonly used in preparations since it attacks glass, quartz, porcelain, and Pt containers at elevated temperatures (greater than 100 °C). However, the presence of 3PO4 will not adversely effect any of the elements at low µg/mL levels and below.
If your matrix is not listed above, consult our Interactive Periodic Table or contact us for assistance.
My Wishlist
Wishlist is empty.
