Trace Analysis Guide - Contamination From the Analyst and Apparatus
- Home
- Trace Analysis Guide - Contamination From the Analyst and Apparatus
- Inorganic Ventures Tech Centre
- Trace Analysis Guide
- Trace Analysis Guide part 10 - Contamination From the Analyst and Apparatus
Overview
Part 8, Environmental Contamination, discussed the importance of a clean room to reduce air contaminants while performing trace analyses. A clean room is equally important for minimizing contamination from the analyst.
Contamination From the Analyst
The area pictured in figure 10.1 is the buffer zone between the outside air and the main clean room. Air entering with the analyst is filtered (at the rate of 1.5 times per minute) before the analyst enters the main clean area. A cart contains a HEPA vacuum used for cleaning objects to be taken into the main clean room. A 'sticky mat' on the floor just inside the room will remove shoe dust and other contaminants. Another sticky mat (not pictured) is placed just before the entry way to the buffer zone.
Fig. 10.1: Entering the Clean Room

The analyst pictured here is wearing disposable "clean room" clothing. There are three separate pieces (head covering, foot covering, and main coverall). The clothing is worn over normal street clothes and should never leave the clean room area.
Common Contaminants
- Sweat contains Ca, Mg, Pb, K, NH4+ , SO4-2, PO4-3, and Cd (for those who smoke).
- Cosmetics can contain high concentrations of Al, Be, Ca, Cu, Cr, K, Fe, Mn, Ti, and Zn.
- Some hair dyes contain Pb(OAC)2.
- Dandruff shampoo can contain significant levels of Se.
- Eye make-up may contain Hg as a preservative.
- Calamine lotion is almost pure ZnO.
- Watches and jewellery contain an assortment of elements and should not be worn in the laboratory.
Analyst Tips
- Keep the volume of reagents to a minimum.
- Keep sample preparation and measurement times to a minimum (use timers and don't let a digestate sit overnight or even during a lunch break).
- Keep a history of beakers and other apparatus as to chemical exposure.
- Exercise care in handling reagents (exposure to atmosphere or pouring liquid back into the same container or into the wrong container can result in serious contamination).
- Disposable powder-free gloves prevent contamination from earlier exposures as opposed to reusable gloves (vinyl is better for difficult manipulations such as pipetting and handling wet glassware while latex is more rugged).
- Disposable laboratory coats prevent contamination from earlier exposures.
- Disposable foot coverings keep any dirt that the 'sticky mat' missed contained.
TIP: Dangerous operations require appropriate laboratory clothing REGARDLESS of the potential for contamination.
Apparatus Contamination
Another potential source of contamination is caused by the apparatus that comes into contact with the sample. Careful planning is required by the analyst as to the type of apparatus that comes into contact with a particular trace metals analysis. All apparatus should be specified in the procedure along with any cleaning procedures or special precautions. Although the type and degree of contamination that occurs when using certain apparatus can be predicted, the importance of one or more blanks with every preparation should be apparent from the following table.
Table 10.1: Results of Spectrochemical Analysis
(Hydrogen Fluoride, Hydrogen Chloride, and Nitric Acid [all high purity] analysed after evaporation in Teflon, Platinum, and Quartz dishes)
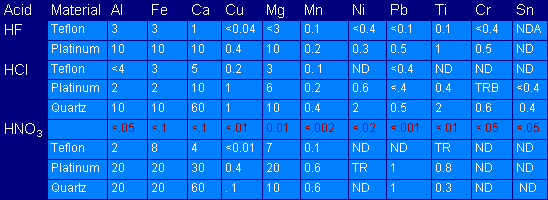
Elements determined, ng/g
ND = Not detected
TR = Trace, not evaluated quantitatively
A Closer Look at Quartz
Quartz is a popular material for apparatuses used in trace elemental analysis. There are two types of quartz: opaque and transparent.
Opaque quartz has the highest trace element concentration and should not be used for trace analysis.
Transparent quartz (types I and II) are made from naturally occurring quartz crystals or sands. Type I is made by electric melting and type II by flame melting. Type II has slightly less impurities than type I (some impurities are volatilized by the flame). Type III quartz is made synthetically by vapor phase hydrolysis of pure silicon compounds such as SiCl4. This type of quartz is more pure than the natural quartz with the exception of Cl - which is ~ 50 ppm. Type IV quartz is synthetically made from SiCl4 using a process involving electrical fusion of the oxidized staring material. It is as pure as type III, with respect to trace metal content, and contains much more Cl-. Use synthetic quartz whenever possible.
Table 10.2 shows typical impurities in natural and synthetic quartz.
Table 10.2: Recorded Impurity Levels in Types of Quartz1

ND = Not detected
Table 10.3 shows a comparison of impurities in natural quartz to other common laboratory apparatus.
Table 10.3: Trace Element Concentration of Container Materials

Figure 10.4 demonstrates how quartz is the cleanest of all sample container materials that can withstand typical ashing temperatures (T > 400 °C).
Figure 10.4: Container Materials in order of Increasing Impurites1
- Polyethylene - Low density (free radical initiated)
- Fluorocarbons - (i.e. - Teflon, Tefzel, Halar, Kel-F)
- Quartz - Synthetic
- Polyethylene - High density
- Quartz - Natural
- Platinum
- Borosilicate
Apparatus Tips
- Avoid filtration due to contamination from the filter paper. It is far better to centrifuge the sample using polyethylene tubes (acid-leached).
- If filtration is necessary, use Teflon filters as your first preference. These are followed by cellulose acetate, polypropylene, or polycarbonate filters that have been acid-leached.
- Avoid rubber stoppers and all tubing except that made from Teflon or polyethylene.
- For grinding, use apparatus made from quartz, tungsten carbide, or pure molybdenum since they introduce significant contamination of only a single element (the tungsten carbide does introduce some cobalt).
- When sieving, use nylon sieves.
- Ovens and muffle furnaces are obvious sources of contamination and should be replaced with flames if at all possible.
- Microwave digestion in a closed Teflon (PFA) digestion vessel offers many obvious advantages over other forms of sample preparation.
1. T. Murphy, National Bureau of Standards Special Publication 422, "Accuracy in Trace Analysis: Sampling, Sample Handling, and Analysis", Proceedings of the 7th IMR Symposium.
- WHAT ARE YOU LOOKING FOR?Search
- Login / Register
- Reference Materials
- Liquid Handling
- Chromatography and Spectroscopy
- Testing
- Labware
- Resources
- Wishlist (0)
- Compare (0)
- Contact Us
